Phthalates: Between Necessity, Risk, and Innovation
Phthalates are chemicals both valued as plasticizers and criticized as endocrine disruptors. Widely used in manufacturing yet raising health concerns, they are difficult to analyze in complex samples like food, cosmetics, and biological material. Standard methods such as GC-MS/MS and LC-MS/MS are essential but limited. This article explains what phthalates are, why they are used, their risks, and how new technologies like SICRIT® may improve analysis.
What Are Phthalates Used for?
Phthalates are a group of chemical compounds most commonly used to soften plastics, making them more flexible and durable. They are found in a wide variety of products: food packaging, flooring, toys, cosmetics, and even medical devices. Without phthalates, many of the plastic-based everyday items we rely on would be less versatile or functional. Their role in stabilizing formulations and extending material life makes them nearly indispensable in modern manufacturing and consumer goods.
The Risks of Phthalates
While phthalates offer benefits in terms of material performance, they also raise serious concerns for health and the environment. Unlike pesticides, phthalates are not directly applied but can migrate from products into air, dust, food, and even the human body. The problematic thing about that is that studies have shown that phthalates can interfere with the endocrine system, leading to potential developmental, reproductive, and metabolic disorders. Children are considered especially vulnerable due to higher relative exposure through toys, household dust, and diet.
From a societal perspective, the widespread use of phthalates makes their avoidance nearly impossible. Everyday exposure happens in diverse settings, and while individual doses may be small, long-term and combined effects remain difficult to assess. The environmental risks are equally pressing. Because phthalates are not chemically bound to plastics, they can leach out over time. When plastic products degrade into microplastics, phthalates are released into soil, rivers, and oceans. In aquatic ecosystems, they can accumulate in sediments and enter the food chain. These disruptions at the organism level influence whole food chains and can translate into broader ecological consequences, such as shifts in species populations and weakened biodiversity.
Regulation and Monitoring of Phthalates
To mitigate risks, many countries have introduced strict regulations on specific phthalates. The European Union, for example, has banned several phthalates in toys, childcare articles, and cosmetics, while restricting their use in food-contact materials. Monitoring and enforcement remain essential since alternatives are not always free of similar concerns.
It becomes obvious that analytical testing plays a crucial role here. Phthalates can be detected and quantified using techniques such as gas or liquid chromatography coupled with mass spectrometry (GC-MS/MS or LC-MS/MS). The challenge, however, lies in the fact that phthalates occur in complex matrices, from plastics to personal care products to biological samples, requiring sensitive as well as reliable methods.
Challenges in Phthalates Analysis
Traditional methods such as GC-MS/MS and LC-MS/MS are well-established for detecting phthalates, and they allow simultaneous analysis of a wide range of compounds. However, these approaches also reveal certain gaps. Many phthalates are relatively volatile and non-polar, which makes them well-suited for GC-based separation. But newer plasticizers, designed as substitutes for regulated phthalates, can be far more polar or thermally unstable. These substances often degrade under high temperatures required in GC systems or are insufficiently ionized in conventional LC-MS workflows. The result: incomplete detection, lower sensitivity, or even false negatives.
Another challenge is the complexity of real-world matrices. As addressed before, phthalates wander from plastics into food, cosmetics, and biological fluids, each containing dozens of interfering compounds. Traditional sample preparation steps, such as solvent extraction and cleanup, may remove not only unwanted background but also part of the target analytes. This can lead to underestimation of exposure levels, particularly when phthalates occur at trace concentrations.
These limitations highlight a gap in comprehensive monitoring: While the main regulated phthalates can be reliably quantified, emerging substitutes and more polar metabolites often escape detection. This means that regulators and researchers may only see part of the picture, making risk assessments less complete.
Closing the Gap by Innovation
Innovative approaches such as the SICRIT® Ionization Technology provide an opportunity to close this gap. By enabling soft ionization of both polar and non-polar phthalates within the same method, SICRIT® broadens the detectable spectrum. This not only simplifies workflows by reducing the need for multiple specialized methods. But it also improves sensitivity for compounds that would otherwise remain hidden. In practice, this translates into faster, more reliable, and more comprehensive phthalate analysis, directly supporting both consumer safety and environmental protection.
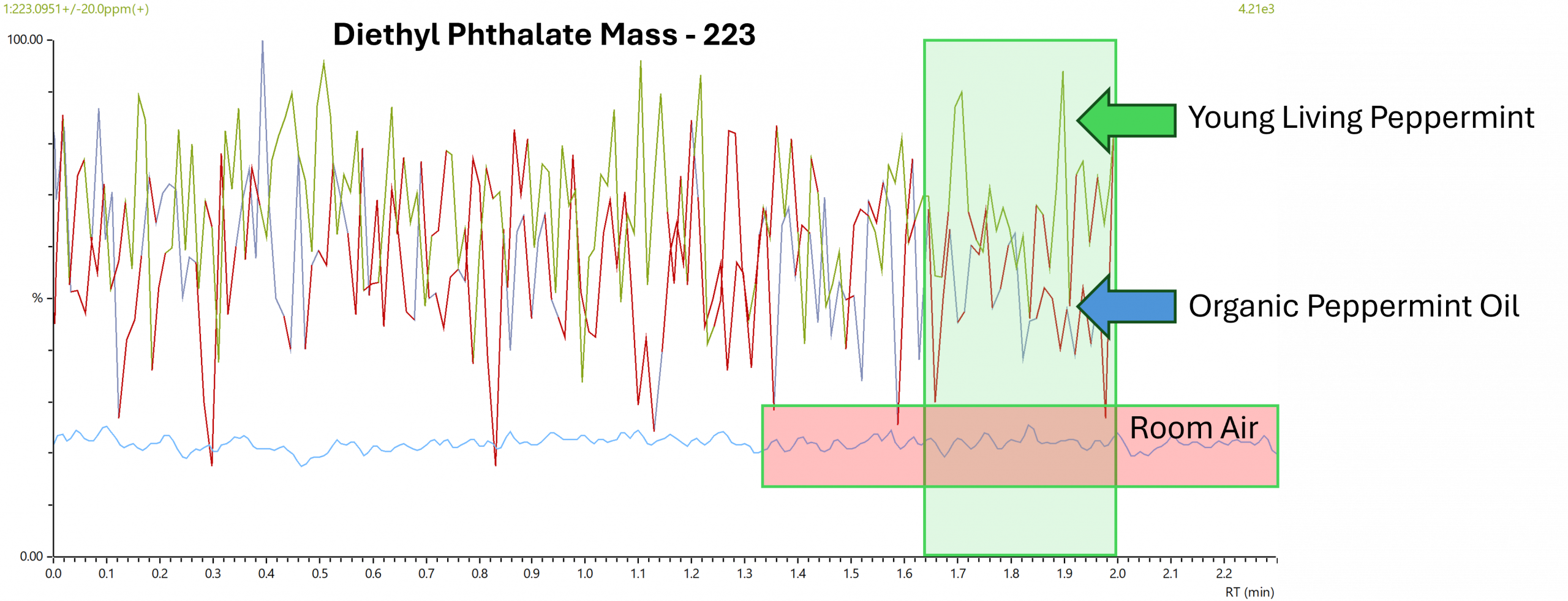
Data from the Plasmion Lab
Measured by Direct Sniffing
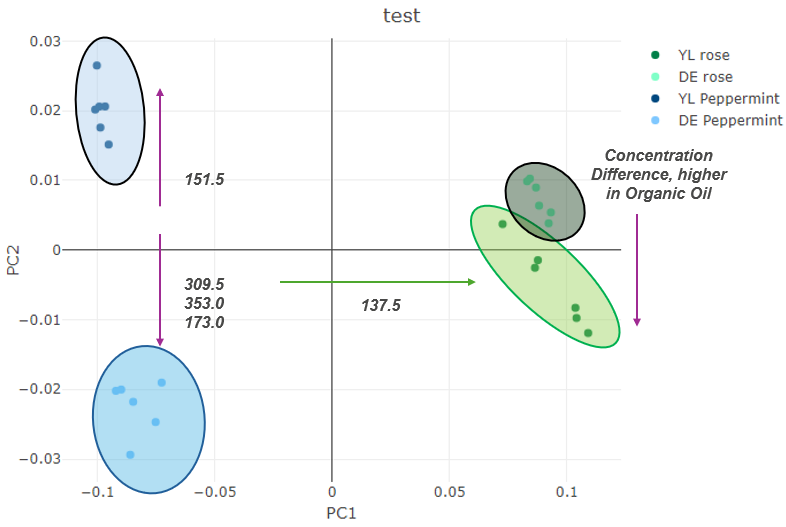
Concentration difference in brands for rosemary oil
Terpene profile difference in the peppermint oil brands
Comparison of phthalate content in room air compared to two different essential oils